Formulation Development
Determination of the most suitable formulation for your product using HTPD (high throughout development).
The use of the formulation plate developed by us, it is possible to evaluate the physical stability of your product in different formulations for the physical and chemical properties of the protein.
With this knowledge, the most suitable formulation can be found and determined specifically for the product to be formulated.
Lentivirus Production
Do you need your lentiviral vector as starting material for cell therapy and/or a lentivirus as a drug product?
Whether you want to develop or produce your lentivirus vector, we can help you!
Our end-to-end CDMO services, spanning vector design to GMP-compliant manufacturing, leverage our proprietary Lenti.RiGHT® packaging and producer cell line. This ensures high titers, stable vectors, and cost-efficient solutions, streamlining your path to seamless scale-up and commercialization.
Development and Manufacturing of Lentiviral Vectors for Cell Therapies
We offer integrated CDMO services to develop and manufacture lentiviral vectors. We can produce the viral vector by transient transfection, packaging, or producer cell line.
As one stop-shop we cover all steps, from vector design and development to process development and GMP manufacturing to analytics, bioassays, QC and fill & finish.
We rely on our Lenti.RiGHT® packaging cell line as a production system to unlock the full potential of your lentiviral vector construct. Choose what works best for you from our flexible development and manufacturing services.
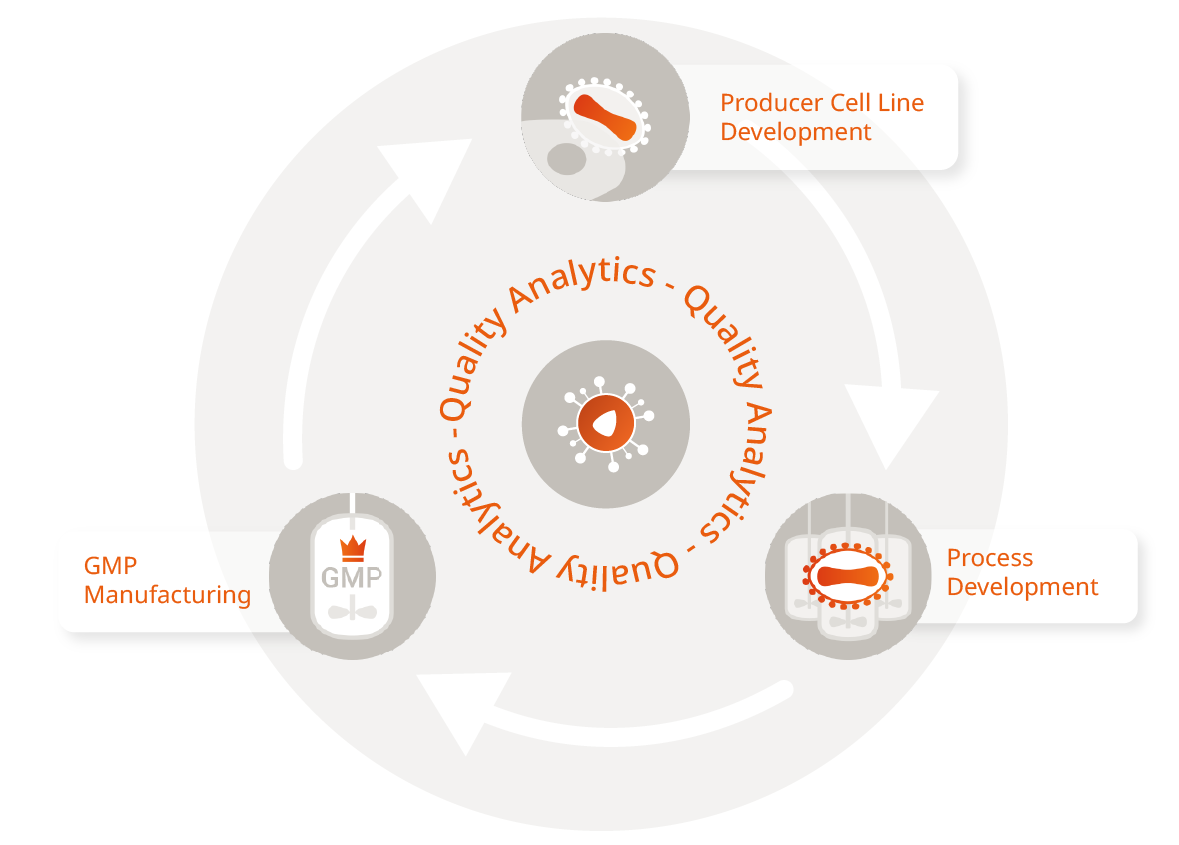
Integrated Services As You Need It
Vector Design and Development
- Lentiviral Production with either transient transfection or Lenti.RiGHT® as a production system
- Stable insertion of the gene of interest (GOI) in combination with DirectedLuck® transposase
- Generation of cell clones capable of producing highest titers
- Testing of genetic stability by performing long-term passaging experiments, including next generation sequencing (NGS) techniques
Process Development (DSP/USP)
- Customized solutions tailored to specific product needs
- Advanced filtration technologies enhance purity and safety
- State-of-the-art chromatographic systems ensure high yield and precise purification
- Process efficiency maximizes recovery and minimizes production time and costs
- Tailor-made purification and formulation process depending on your needs
- Lentiviral production development in various scales and reactor types, including but not limited to wave, orbital shaken, and stirred tank bioreactors
(GMP) Manufacturing*
- Versatility in scale-up supports small to large-scale production (up to 200L scale in stirred tank bioreactors)
- Reactor flexibility adapts to different bioreactor types for optimal production
- Process robustness maintains high yield and quality across scales and reactor types
- Manufacturing of drug substance and drug product fulfilling requirements specified in EU GMP Annex 1
*Coming Soon
Quality Control & Analytics
- Functional titer: vector functionality ensured by detection of provirus integration by ddPCR
- Gene product expression: assessed at protein (flow cytometry) and RNA (ddPCR) levels
- Physical particle titer: measured by p24 Gag ELISA for accurate viral particle count
- Physical genomic particle titer: quantified by ddPCR or RT-PCR to assess genomic RNA
- RCL detection: ensures the absence of replication-competent lentiviruses
- Integration profile: analyzed by NGS for comprehensive safety and predictability
- Sterility test: ensures sterility of production runs
- Host cell DNA and protein assays: Minimal residuals ensured by quantification residuals
- Mycoplasma and endotoxin tests: ensure absence of contaminants and low endotoxin levels
Which System is RiGHT?
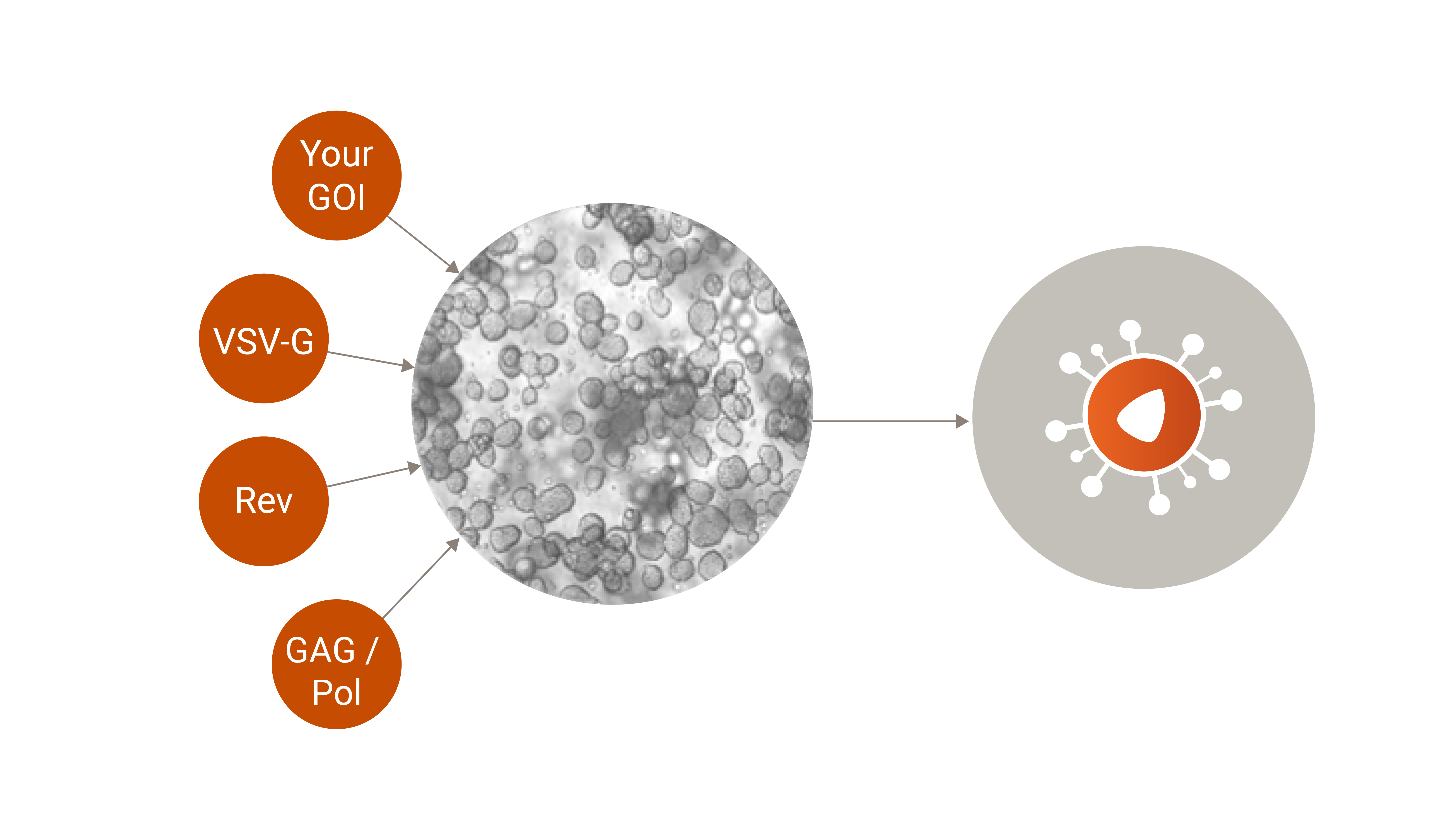
Transient Transfections for Lentiviral Vector Production
Ideal Use Case: Early Phase Clinical Trial
- Rapid Production Cycle: Transient transfection allows for the rapid production of lentiviral vectors, making it ideal for applications requiring quick turnaround times, such as early-phase (pre)clinical trials or urgent research needs.
- Flexibility: This method provides high flexibility in changing vector designs and when incorporating genetic modifications. Researchers can easily adapt to different constructs without the need for long development cycles.
- Scalability: Though traditionally seen as less scalable than stable cell lines, advancements in transient transfection techniques have significantly improved yields, making them feasible for larger-scale productions.
- Reduced Development Time: Bypassing the need for generating stable cell lines cuts down on the time required to initiate production. This allows for faster project initiation and delivery.
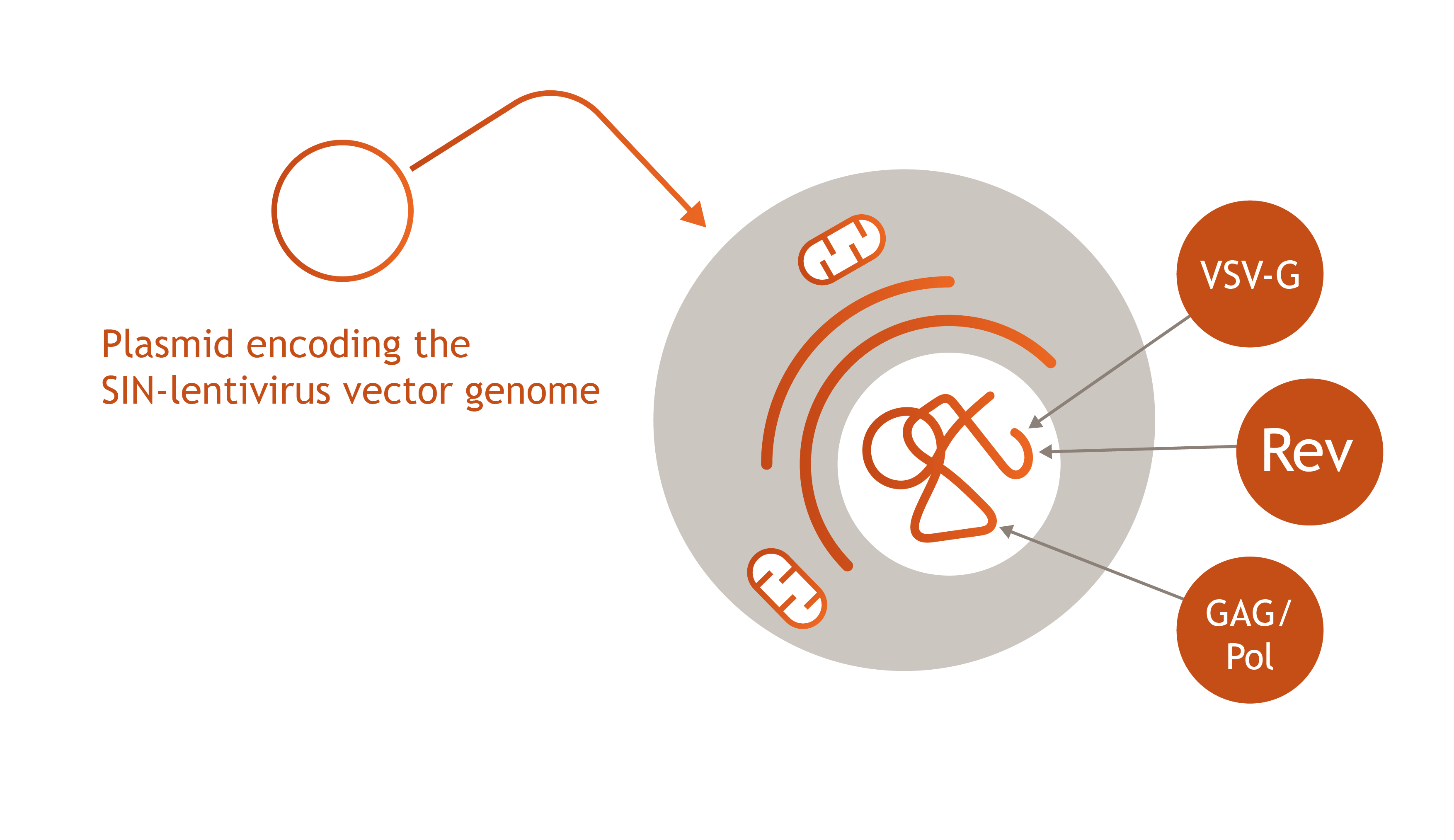
Lenti.RiGHT® Packaging Cell Line
Ideal Use Case: High-Titer Viral Production for Research and Early Clinical Studies
- Flexibility in Vector Design: Packaging cells without the stable integration of the lentiviral transfer vector allow for quick changes to the transfer vector, thereby providing adaptability for different therapeutic applications.
- Streamlined Process: This approach simplifies the production process by focusing on the generation of high-titer viral particles without the complications involved with integrating the lentiviral transfer vector into the packaging cell line.
- High-Titer Production: These cells are optimized for high-titer viral production, making them suitable for producing sufficient quantities of vectors for research and early clinical studies.
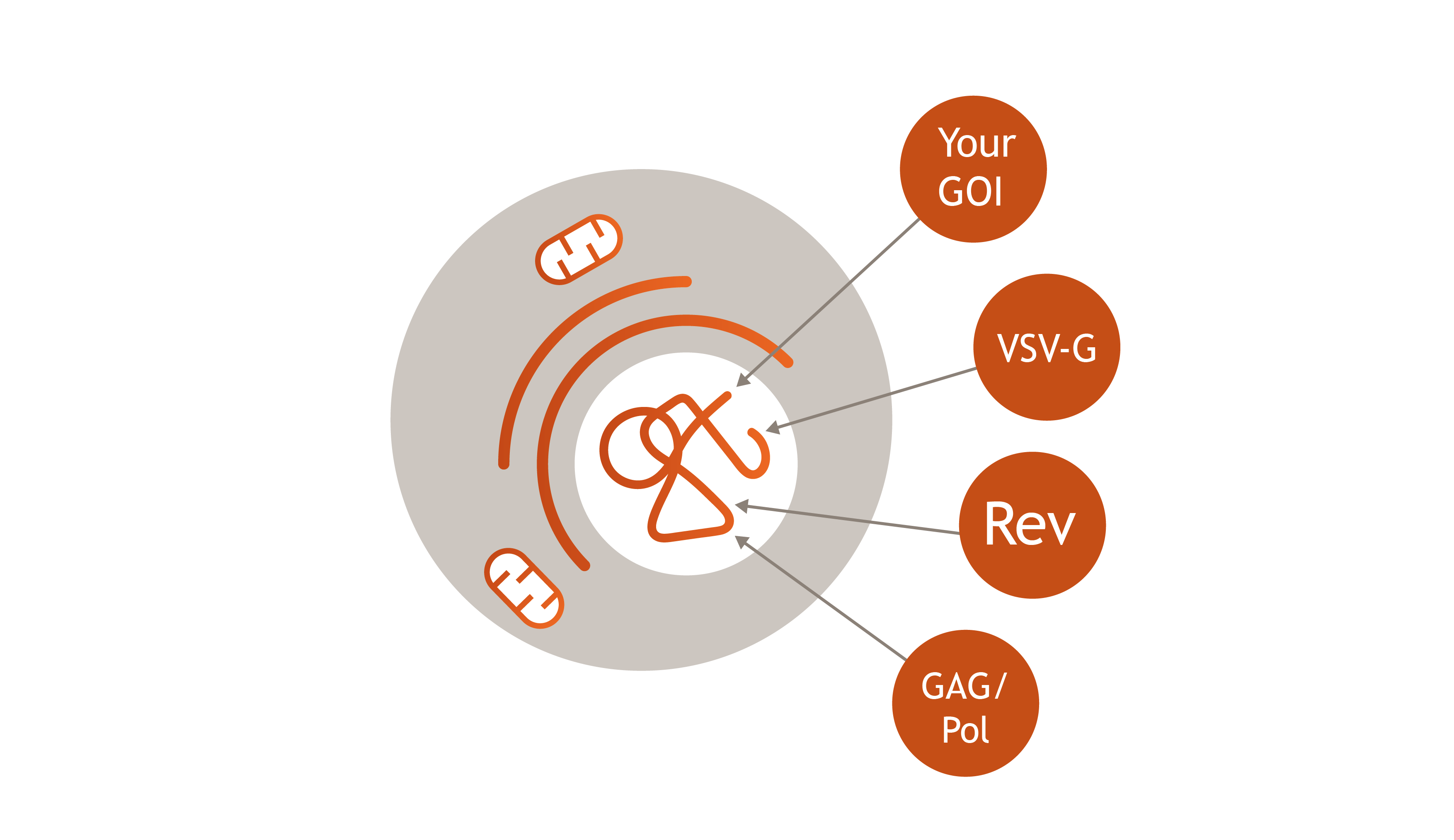
Lenti.RiGHT® Producer Cell Line
Ideal Use Case: Later-Phase Clinical Trials and Commercial Production- Consistency and Reproducibility: With all lentiviral packaging genes and the transfer vector stably integrated, these production cells ensure consistent vector production, which is critical for clinical applications where batch-to-batch variability must be minimized.
- High Yield and Efficiency: These cells are engineered for optimal expression of all necessary components, which leads to high-yield production suitable for the large-scale manufacturing needed in later-phase clinical trials and commercial production.
- Simplified Manufacturing: The stable integration of all components streamlines the manufacturing process, thereby reducing the complexity and variability associated with transient transfections and ensuring a more robust production pipeline.
- Cost-Effectiveness: Over time, the use of these stable production cell lines can be more cost-effective thanks to reduced variability, elimination of the need for repeated transfections, and consistently high yields. This all leads to lower overall production costs.
Lenti.RiGHT® Production System
The Lenti.RiGHT® packaging and producer cell line is well suited for the efficient and reliable production of lentiviral vectors. This lentivirus production platform is based on our GMP-grade HEK293 suspension cell, which is stably transfected with all required viral components. The inducible system enables a scalable and efficient production of high-titer lentiviral particles for various applications starting from early in vitro demonstration testing and preclinical studies through to gene and cell therapies in a clinical environment.
Rely High Titers and Stable Vectors
- Proprietary and GMP compliant HEK293 cell line laid down as master cell bank for reliable and consistent vector production
- Optimized transposase system ensuring high titers and genetic stability
- Tight regulatory system for precise induction of packaging genes
Save Time and Money
- Streamlined processes for fast turnaround and cost-efficiency
- Scalable solutions tailored to your project's specific needs
- Comprehensive analytics panel to reduce development time and ensure quality
Get Ready for Scale Up and Commercialization
- Proven upstream and downstream processes, scalable up to 200L
- Seamless transition from research to GMP-grade production
- Expertise in regulatory requirements and commercial readiness
Elevate Your Candidate With The Right CDMO
Explore Related Content
Newsletter
Join for ProBioGen News - make sure to be always up-to-date and fully informed with our latest company announcements.Rely on Fast Timelines
- Early focus on process friendliness for cell lines and media use eliminates risk of late surprises
- One-stop-shop with integrated services and technologies
- Paralell development of multiple molecules possible
Get Your High Quality Candidate
- Strong expertise in CLD and with complex molecules
- Stable and high performing molecules
- Long standing expertise and hundrets of satisfied customers in end-to-end projects
Have the Flexibility You Need
- Experienced inhouse CMC-Managers as individual client interface
- Customized and modular approach allows for outstanding flexibility
- Proven passion for customer molecules and projects





