Lenti.RiGHT® Production System
Rely on our Lenti.RiGHT® packaging and producer cell line as your lentivirus production system and unlock the full potential of your lentiviral vector construct. In addition to the production of lentiviral particles by means of transient transfections, this represents an exciting alternative with several advantages. The choice is yours.
Packaging and Producer Cell Line for Lentiviruses
The Lenti.RiGHT® packaging and producer cell lines are well suited for the reliable and efficient production of lentiviral vectors. Our lentivirus production platform is based on our GMP-grade HEK293 suspension cell, which is stably transfected with all required viral components. The inducible system enables the scalable and efficient production of high-titer lentiviral particles for various applications, starting from early in vitro demonstration testing and preclinical studies and continuing through to gene and cell therapies in a clinical environment.

Key Features of Lenti.RiGHT®
Based on ProBioGen's suspension cell line HEK293, which exhibits superior growth characteristics in chemically-defined media that allows easy scale-up
Unique packaging cell clone with inducible gag-pol, rev and VSV-G genes located in best-suited genomic spots
Proven long-term stability of Lenti.RiGHT® packaging cells thanks to a stringent regulation system
Choose your RiGHT Development Procedure
Our Promise to You
Rely on Fast Timelines
- One-Stop-Shop with seamless, integrated Services
- Parallel Processes
- Efficient project execution
- Do it right from the beginning
Get Your High Quality Candidate
- Scientific excellence
- Deep understanding for molecule / Long + broad experience
- Strong Analytics teams and stellar quality management services
- Enabling technologies
Have the Flexibility You Need
- Modular Offering
- Customer Centricity/ Strong Partnership Approach
- Experienced Project Managers and efficient decision-making
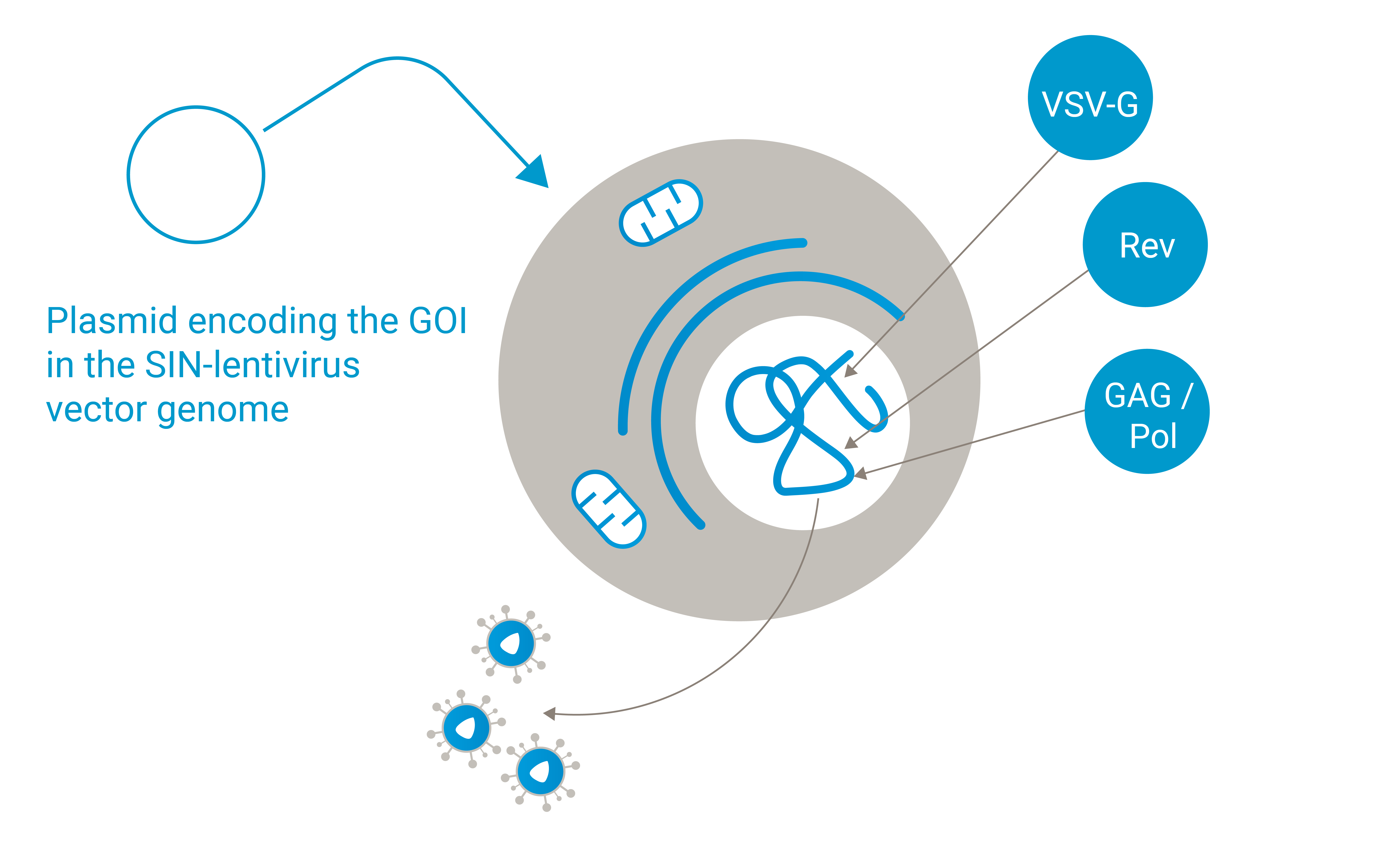
Ideal use case: Lenti.RiGHT® is well suited for high-titer viral production for research and early clinical studies.
Flexibility in vector design: Packaging cells without the stable integration of the lentiviral transfer vector allow for quick changes to the transfer vector, making it adaptable for different therapeutic applications.
Streamlined process: This approach simplifies the production process by focusing on the generation of high-titer viral particles without the complications associated with integrating the lentiviral transfer vector into the packaging cell line.
High-titer production: These cells are optimized for high-titer viral production, making them suitable for producing sufficient quantities of vectors for research and early clinical studies.
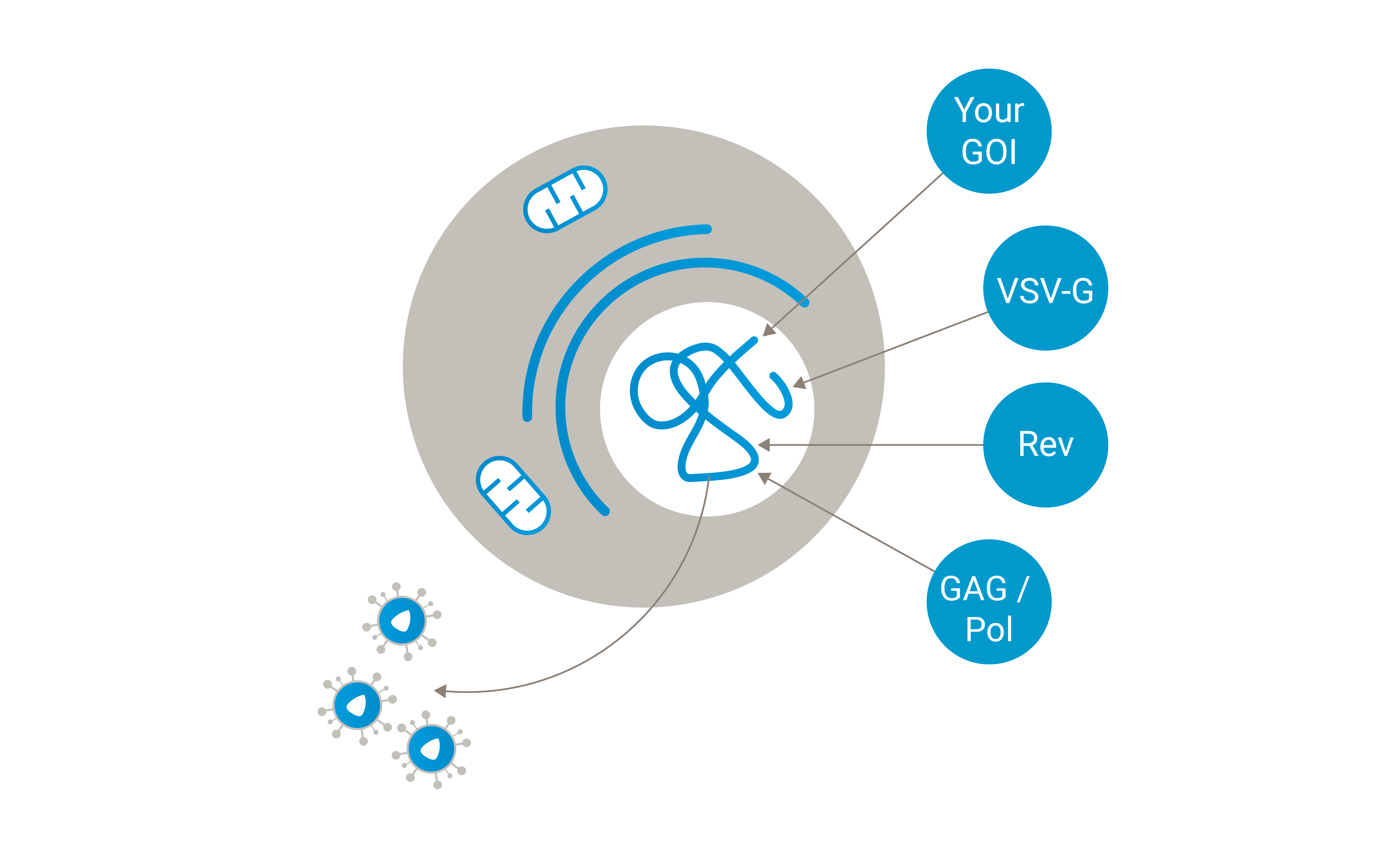
Ideal use case: Lenti.RiGHT® is ideally suited for later-phase clinical trials and commercial production.
Consistency and reproducibility: With all lentiviral packaging genes and the transfer vector stably integrated, these production cells ensure consistent vector production, which is critical for clinical applications where batch-to-batch variability must be minimized.
High yield and efficiency: These cells are engineered for optimal expression of all necessary components, leading to high-yield production suitable for the large-scale manufacturing that will be required in later-phase clinical trials and commercial production.
Simplified manufacturing: The stable integration of all components streamlines the manufacturing process, thereby reducing the complexity and variability associated with transient transfections and ensuring a more robust production pipeline. Initial and early material can be provided from clone pool production.
Cost-effectiveness: Over time, the use of these stable production cell lines can be more cost-effective due to reduced variability, elimination of the need for repeated transfections, and consistent high yields, leading to lower overall production costs.
From Gene to Lead Candidate

Our Promise To You
Efficient and Cost-Saving Production Cell Lines
- No transfection reagents required
- No GMP-grade plasmids required
- Early material access by using clone pool production or transient expression
Safety Secured
- GMP-qualified starter cell bank
- Modification/single-cell cloning w/o animal components
- Fully-documented history, including HEK pre-GMP history
Robust, Stable, and Scalable
- Proven cell line stability
- Robust growth without lentivirus release
- Straightforward scale-up and purification
V2 Benefits- TBD



-
One-Stop-Shop with seamles, integrated Services
-
Parallel Processes
-
Efficient project execution
-
Do it right from the beginning
-
Scientific excellence
-
Deep understanding for molecule / Long + broad experience
-
Strong Analytics teams and stellar quality management services
-
Enabling technologies
-
Modular Offering
-
Customer Centricity/ Strong Partnership Approach
-
Experienced Project Managers and efficient decision making




