Formulation Development
Finding the most suitable formulation for your product is essential. Having the right formulation will help ensure the stability of your product and can reduce side effects when administered to the patient.
We are excited to offer our new formulation development service to help you find optimal buffer solutions or enhance existing formulations with various additives. Our expert team will assist in selecting and applying the right additives to boost your products' efficiency and stability.
Formulation Development Services for Your Product
At ProBioGen, we have developed a multi-phase strategy for identifying the optimal buffer for your product. A range of analyses and stress conditions are performed to thoroughly evaluate the stability of your product.
We use high-throughput development (HTPD) to achieve the most suitable buffer for your product.
For projects ranging from cell line development to manufacturing, we start formulation development as early as possible in order to achieve the shortest timelines. However, we also offer our high-throughput formulation development as a stand-alone service.
Integrated Development
Formulation development runs in parallel with process development, so that all knowledge that has been obtained can be transferred quickly and adaptations can be implemented directly in process development or formulation development that are specific to the product.
High-Throughput Formulation Screening
We developed a formulation concept that uses nanoDSF (conformational stability) and DLS (colloidal stability) by Prometheus Panta to simultaneously test different formulations for stability during an initial screening phase.
Different molarities of the excipients, additives, and pH values are tested during this process. The screening is performed with a low product concentration purified by ProteinA chromatography.
In-Depth Characterization
Based on the data collected, the best base buffer is tested more intensively in a more detailed screening process (additional analysis, e. g. size exclusion chromatography and initial freeze thaw cycles and additional additives for stabilization).
Best-in-Class Selection
During the screening, the buffer candidates for the stability study (formulation assessment) are determined as well as the final optimal formulation, concentration and storage conditions for your product.
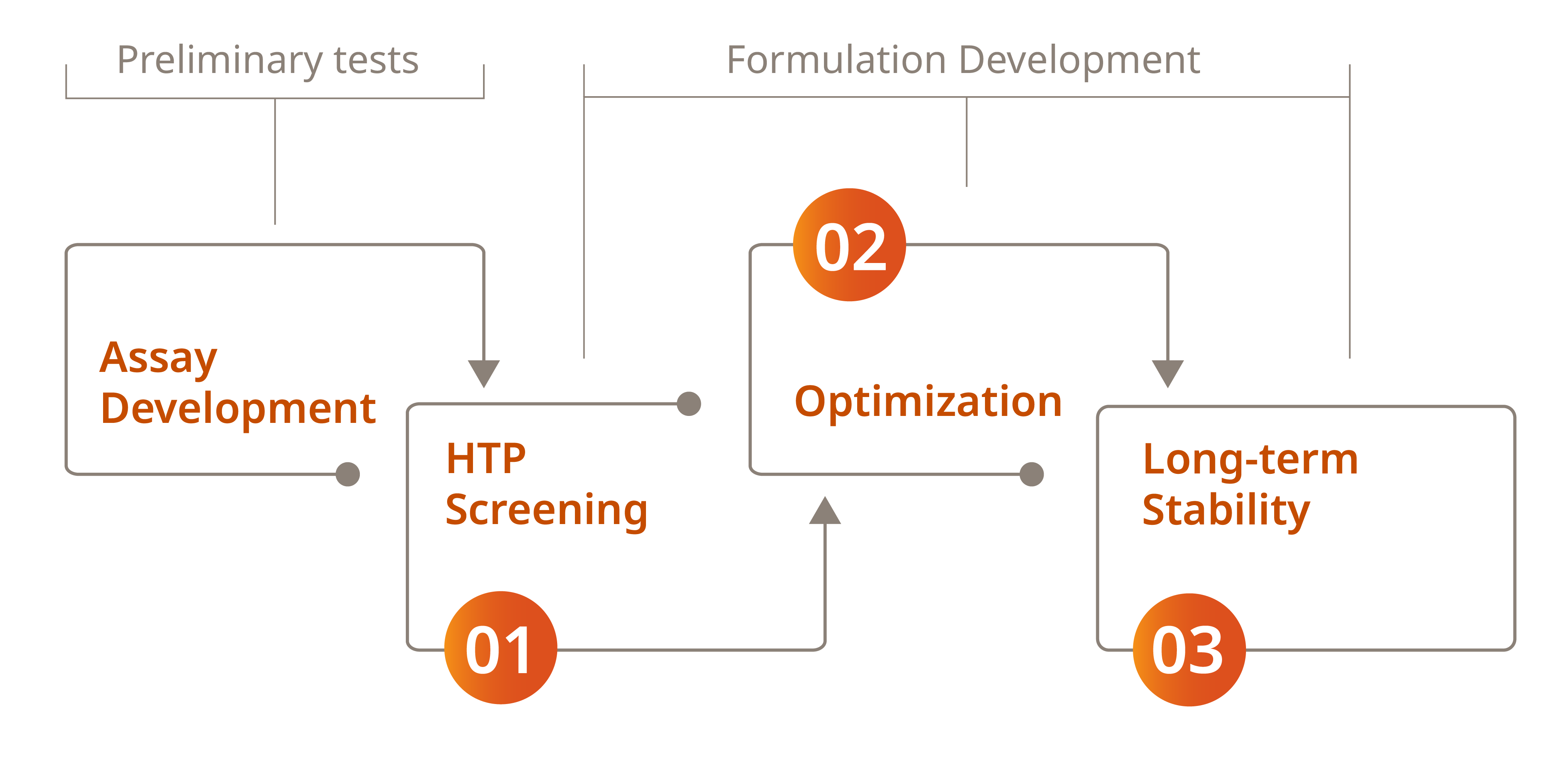
Our Multi-Phase Formulation Development Process
A Process You Can Rely On
Assays Development Phase
Before starting the main phases of formulation development, critical assays are carried out to gain a thorough understanding of the product's properties.
Phase 1 (HTP Screening)
Using design of experiments (DoE), we explore various parameters, including buffer composition, additives, and pH values. This involves testing multiple concentrations of FDA-approved buffer components and additives, along with different pH levels. Detailed evaluations will be performed on at least two buffer systems to determine their overall performance and effectiveness.
Phase 2 (Optimization)
After identifying the best buffer system in phase 1, phase 2 focuses on refinement and optimization. Using DoE, up to four factors are tested, with the base buffer enhanced by additives to assess stability.
Once the optimal formulation is identified, it is subjected to a confirmation run with higher product concentrations (up to 2 levels), based on customer specifications for the final drug product concentration.
Phase 3 (Long-term stability)
Phase 3 is conducted to:
(a) assess how different storage conditions and stress tests affect degradation
(b) determine the best drug substance formulation and long-term storage conditions. While forced degradation studies use harsh conditions and short durations, the insights gained are crucial for understanding real-time impacts and validating degradation detection methods.
Scouting > Screening > Assessment

Our Promise To You
Our Promise to You
Rely on Fast Timelines
- One-Stop-Shop with seamless, integrated Services
- Parallel Processes
- Efficient project execution
- Do it right from the beginning
Get Your High Quality Candidate
- Scientific excellence
- Deep understanding for molecule / Long + broad experience
- Strong Analytics teams and stellar quality management services
- Enabling technologies
Have the Flexibility You Need
- Modular Offering
- Customer Centricity/ Strong Partnership Approach
- Experienced Project Managers and efficient decision-making

-
One-Stop-Shop with seamles, integrated Services
Parallel Processes
-
Efficient project execution
-
Do it right from the beginning

-
Scientific excellence
-
Deep understanding for molecule / Long + broad experience
-
Strong Analytics teams and stellar quality management services
-
Enabling technologies

-
Modular Offering
-
Customer Centricity/ Strong Partnership Approach
-
Experienced Project Managers and efficient decision making
Customized Solutions for Your Needs
- We develop formulations specifically adapted to the needs and requirements of your product
- Our in-house development empowers us to quickly test, refine, and launch formulations, ensuring faster time-to-market and greater flexibility.
Higher Quality and Control
- Through in-house development, we ensure complete control and consistently high quality at every stage
- We use the latest technologies to ensure products meet the highest standards of functionality, sustainability, and performance
Expertise & Experience
- Our team offers deep expertise in formulation science and biostatistical evaluation to develop optimal solutions




