Designer Cell Line AGE1.CR.plX®
If you're looking for a high performing avian cell line for vaccine viruses, our immortalized and stable proliferating avian cell line AGE1.CR.pIX® is your ideal solution.
The cell line is fully developed under GMP-compliance and is completely documented.
Highly Permissive Designer Cell Line for Vaccine Viruses
ProBioGen has designed and developed an immortalized and stable proliferating avian cell line, the AGE1.CR.pIX®, that is highly permissive for a variety of different vaccine viruses, including poxvirus vectors. The cell line development, from egg to generation of cell banks, was performed completely under GMP-compliance at ProBioGen and has been fully documented. We developed a chemically-defined medium that allows optimal growth. The cell line is available both as an adherent and as a suspension cell line and is well known in the academic and industrial community.

Key Features of AGE1.CR.PIX®
Fully-documented and free of adventitious agents, including active endogenous retroviruses
Robust and fully-scalable proliferation in suspension culture for different bioreactor systems in chemically-defined media
Highly susceptible to a range of viruses, such as pox-, adeno-, orthomyxo-, rhabdo-, paramyxo- and togaviruses
Readily transferable for flexible, local GMP manufacturing with a small footprint
Cost-saving over manufacturing in CEFs
Suspension USP and Culture Media
The AGE1.CR.pIX® is adapted for suspension culture. The chemically-defined culture media is also available in a powder format to ensure long shelf life, smart storage, and supply chain features.
Cell Banks and Safety
Master cell banks (MCB) and working cell banks (WCB) are available. Extensive safety testing in vitro and in vivo has revealed no adventitious agents. This safety profile is confirmed by transcriptome analysis against pathogen databases. There are no known or new viral species present in the AGE1.CR.pIX® cell line.
AGE1 Workhorse
AGE1.CR.pIX® is a universal platform for a variety of viral vectors and vaccine manufacturing, e. g. gene-therapeutical vectors, oncolytic viruses, and attenuated and vectored vaccines to prevent and cure diseases. It is a key to cost-effective and potent medical interventions.
Use Cases
AGE1.CR.pIX® has demonstrated highly beneficial vaccine production properties, also beyond MVA. A variety of viruses, viral vectors, and vaccine strains have been shown to replicate in AGE1.CR.pIX®, including poxviruses such as:
- Wildtype and vectored MVA
- Fowlpox vectors and veterinary fowlpox virus
- Canarypox strain ALVAC
- Oncolytic and attenuated vaccinia virus derivatives and vectored vaccines such as chimeric alphavirus vectors (with AGE1.CR.pIX-derived helper cell lines)
- Egg drop syndrome virus (EDSV)
- Marek's disease virus (MDV) and the vaccine strain HVT
- Muscovy duck parvovirus (MDPV)
- Influenza A and B strains, also temperature-sensitive live vaccine strains
- Infectious bursal disease virus (IBDV)
- Infectious bronchitis virus (IBV)
- Rabies virus (RABV) and vesicular stomatitis virus (VSV)
And many more.
Our Promise to You
Rely on Fast Timelines
- One-Stop-Shop with seamless, integrated Services
- Parallel Processes
- Efficient project execution
- Do it right from the beginning
Get Your High Quality Candidate
- Scientific excellence
- Deep understanding for molecule / Long + broad experience
- Strong Analytics teams and stellar quality management services
- Enabling technologies
Have the Flexibility You Need
- Modular Offering
- Customer Centricity/ Strong Partnership Approach
- Experienced Project Managers and efficient decision-making
From Gene to Lead Candidate

A Large Variety of Viruses, Viral Vectors and Vaccine Strains Replicate In AGE1.CR.PIX®
AGE1.CR.pIX® has been demonstrated highly beneficial vaccine production properties also beyond MVA. A variety of viruses, viral vectors and vaccine strains have been proved to replicate in AGE1.CR.pIX® include poxviruses such as:
Wildtype and vectored MVA aaaaaaaaaa aaaaaaaaaa
Fowlpox vectors and veterinary fowlpox virus
Canarypox strain ALVAC aaaaaaaaaa aaaaaaaaaa
Egg drop syndrome virus (EDSV)
Infectious bronchitis virus (IBV)
aaaaaaaaaa aaaaaaaaa
Muscovy duck parvovirus (MDPV)
aaaaaaaaaa aaaaaaaaaa
Influenza A and B strains, also temperature-sensitive live vaccine strains
Infectious bursal disease virus (IBDV)
aaaaaaaaaa aaaaaaaaaa
Marek's disease virus (MDV) and the vaccine strain HVT
aaaaaaaaaa aaaaaaaaaaaaaaaaaaaa
Rabies virus (RABV) and vesicular stomatitis virus (VSV)
aaaaaaaaaa aaaaaaaaaaaaaaaaaaaa
Oncolytic and attenuated vaccinia virus derivatives, vectored vaccines such as chimeric alphavirus vectors
Infographic
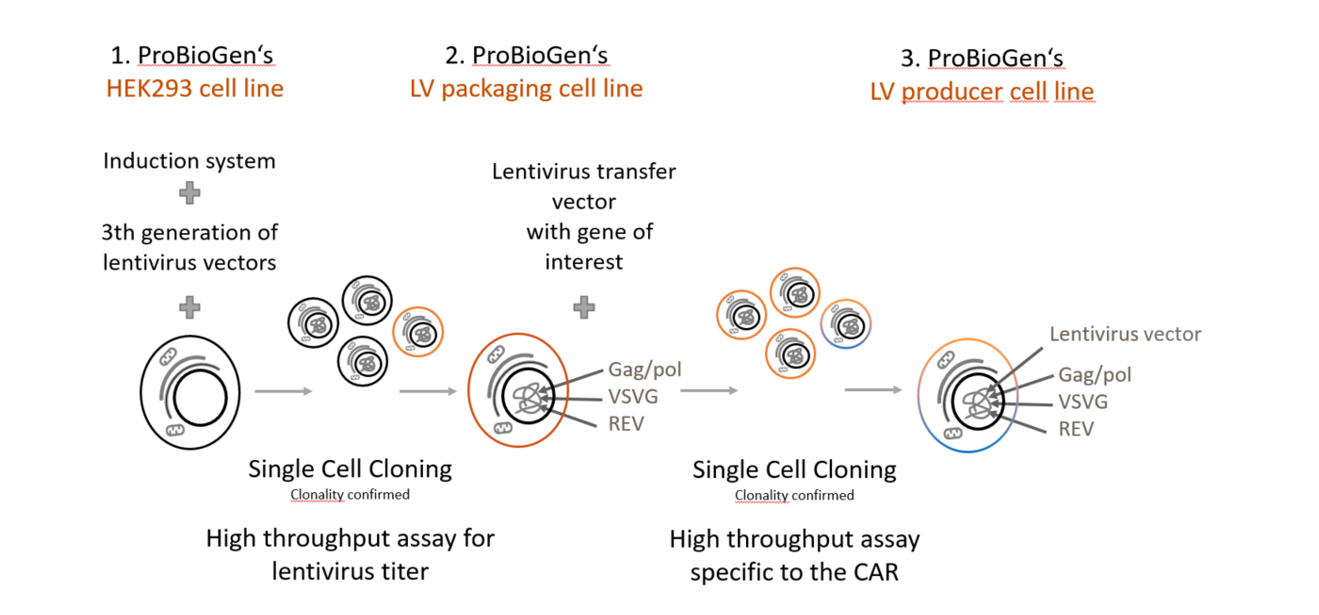
Our Promise To You
Highly Permissive Cell Line
- For a large variety of viruses
- For both human and animal vaccine production, and vectors
Highly Evolved Package
- Chemically-defined media optimized for cell line, also in powder format
- Adherent and suspension cultivation possible
- Comprehensive technology transfer for production at desired scale and purpose
Full Documentation
- From origin, treatments, passaging and raw materials; we confirm that MCBs/WCBs are free of adventitious agents and any reverse transcriptase activity
- Fully GMP compliant (EU and US guidelines)
V2 Benefits- TBD



-
One-Stop-Shop with seamles, integrated Services
-
Parallel Processes
-
Efficient project execution
-
Do it right from the beginning
-
Scientific excellence
-
Deep understanding for molecule / Long + broad experience
-
Strong Analytics teams and stellar quality management services
-
Enabling technologies
-
Modular Offering
-
Customer Centricity/ Strong Partnership Approach
-
Experienced Project Managers and efficient decision making




