Quality Systems for CDMO Projects
Do you want GMP-compliant drug development?
At ProBioGen, we ensure pragmatic solutions tailored to your needs. Benefit from our holistic GMP-compliant quality system, our hands-on approach, and unwavering commitment to excellence.
Quality Systems to Ensure Regulatory Compliance
ProBioGen holds manufacturing licenses and GMP certificates for its manufacturing facilities. We see the challenges posed to our pharmaceutical quality system by regular health authority inspections and customer audits as an opportunity to continuously improve. Our focus is on a holistic approach to regulatory requirements for your product and service.

mABs

BsMABs

Complex Recombinant
Proteins

Fusion Proteins
Cytokines

Clotting Factors
Fully GMP Compliant
As our quality assurance (QA) is involved from the beginning, all tasks, decisions, procedures e. g. follow quality standards and comply with GMP requirements.Quality First
Our quality approach ensures that issues are identified and addressed quickly leading to transparent management of these challenges and finally better solutions.
Hands-on and Pragmatic
Our hands-on and pragmatic approach to quality questions ensures effective communication between you and the project team. You will get firsthand experience into how we work and how issues are managed.With a QP in Charge
Our in-house Qualified Person (QP) is responsible for certifying batches of drug substance for release and actively participates in tasks and projects. Accordingly, you benefit from short distances.
How We Work in Our Quality Unit
Our QA Team
 Our QA team is actively involved in tasks and projects, you can be ensured that thingsare done correctly and in a timely manner.
Our QA team is actively involved in tasks and projects, you can be ensured that thingsare done correctly and in a timely manner.
Our Quality Approach
 Our quality approach ensures that issues are identified and addressed quickly leading totransparent management of these challenges and finally better solutions.
Our quality approach ensures that issues are identified and addressed quickly leading totransparent management of these challenges and finally better solutions.
Our Hands-on and Pragmatic Approach
Our hands-on and pragmatic approach also in  quality questions ensures an effective communicate within the project team and you, giving you firsthand experience of how the work is done and issues are managed.
quality questions ensures an effective communicate within the project team and you, giving you firsthand experience of how the work is done and issues are managed.
Our In-house Qualified Person

Elevate your Molecule - in Time and Quality

Rely on Fast Timelines
- One-Stop-Shop with seamles, integrated Services
- Parallel Processes
- Efficient project execution
- Do it right from the beginning

Get Your High Quality Candidate
- Scientific excellence
- Deep understanding for molecule / Long + broad experience
- Strong Analytics teams and stellar quality management services
- Enabling technologies

Have the Flexibility You Need
- Modular Offering
- Customer Centricity/ Strong Partnership Approach
- Experienced Project Managers and efficient decision making
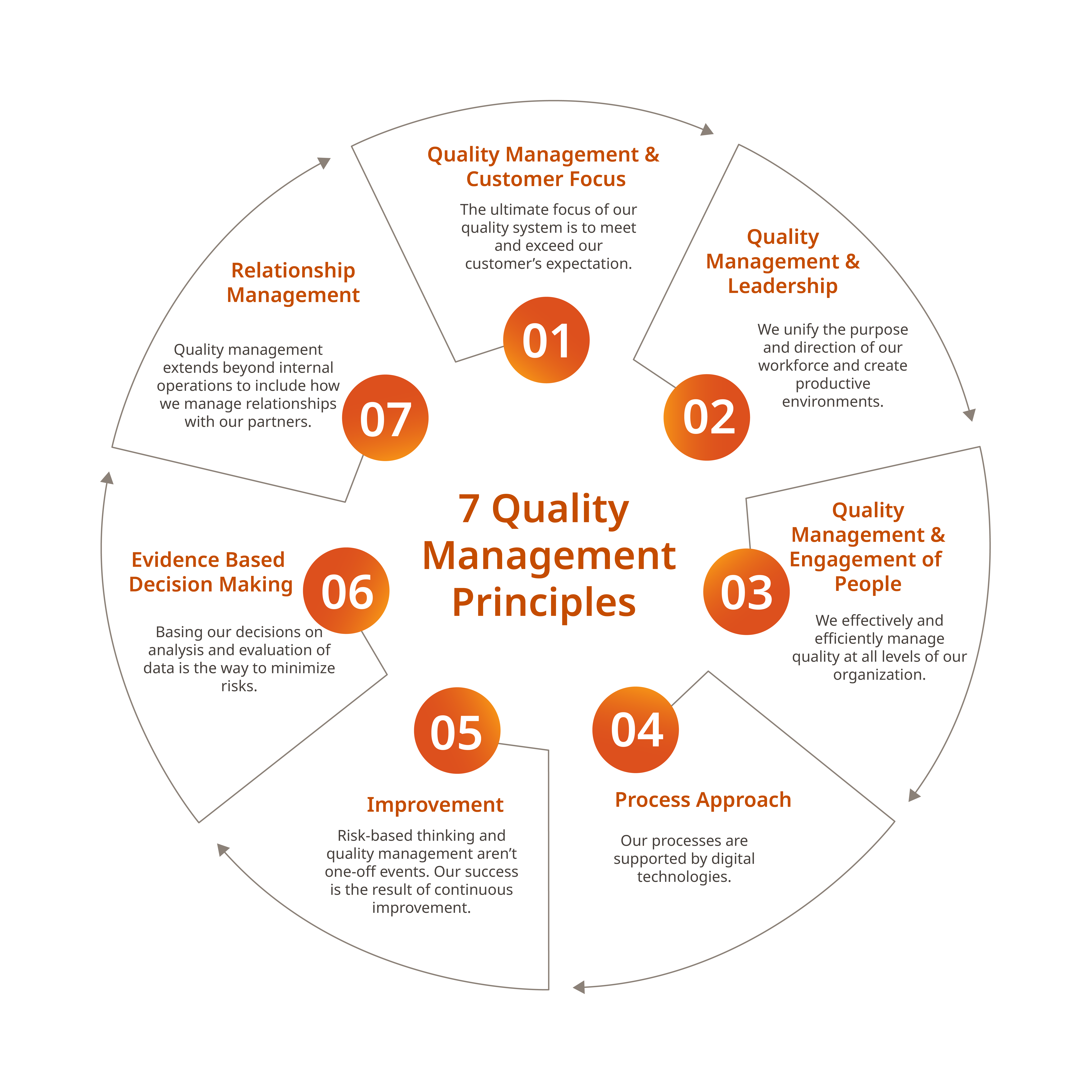
Our Quality Principles
ProBioGen complies with GMP requirements. Our pharmaceutical quality system incorporates appropriate risk management principles, including the use of applicable tools.
QMS Certificates for our Herbert Bayer Str. facilities
QMS Certificates for our Goethe Str. facilities
Our Promise To You
GMP-Compliant
- For manufacturing and testing drug substances including stability studies
- For testing investigational but also commercial drug products
- For importing drug products for clinical trials in Europe
Pragmatic and Competent QA Support
- Guaranteed by our experienced and actively involved QA team
- Guided by an in-house Qualified Person
Quality You Expect
- From our QA team, which is actively involved in projects from the very beginning
Elevate Your Candidate With The Right CDMO
Explore Related Content
Newsletter
Join for ProBioGen News - make sure to be always up-to-date and fully informed with our latest company announcements.Related Technologies

Genetic Glyco-Engineering / ADCC (GlymaxX®) aaaaaaaaaa

DirectedLuck® Transposase with Epigenetic Targeting

Lenti.RiGHT® Packaging and Producer Cell Line aaaaaaa














