Cell Banking (MCBs & WCBs)
Is your cell line ready for manufacture?
We generate GMP-compliant master and working cell banks with precision. Our team produces up to 300 vials in GMP-certified clean rooms, conducting clone-specific testing and delivering data optimized for seamless IND/IMPD filing so you can get your product to market faster.
GMP-Compliant Cell Banking
We produce master cell banks (MCBs) as well as working cell banks (WCBs). We start by checking for clone-specific cell growth and resistance to DMSO during the filling process. Based on the collected data, a GMP-compliant manufacturing procedure and a master batch record are written. In order to protect the interests of the customer, a QAA (quality assurance agreement) is drawn up that describes the responsibilities and the qualitative framework.

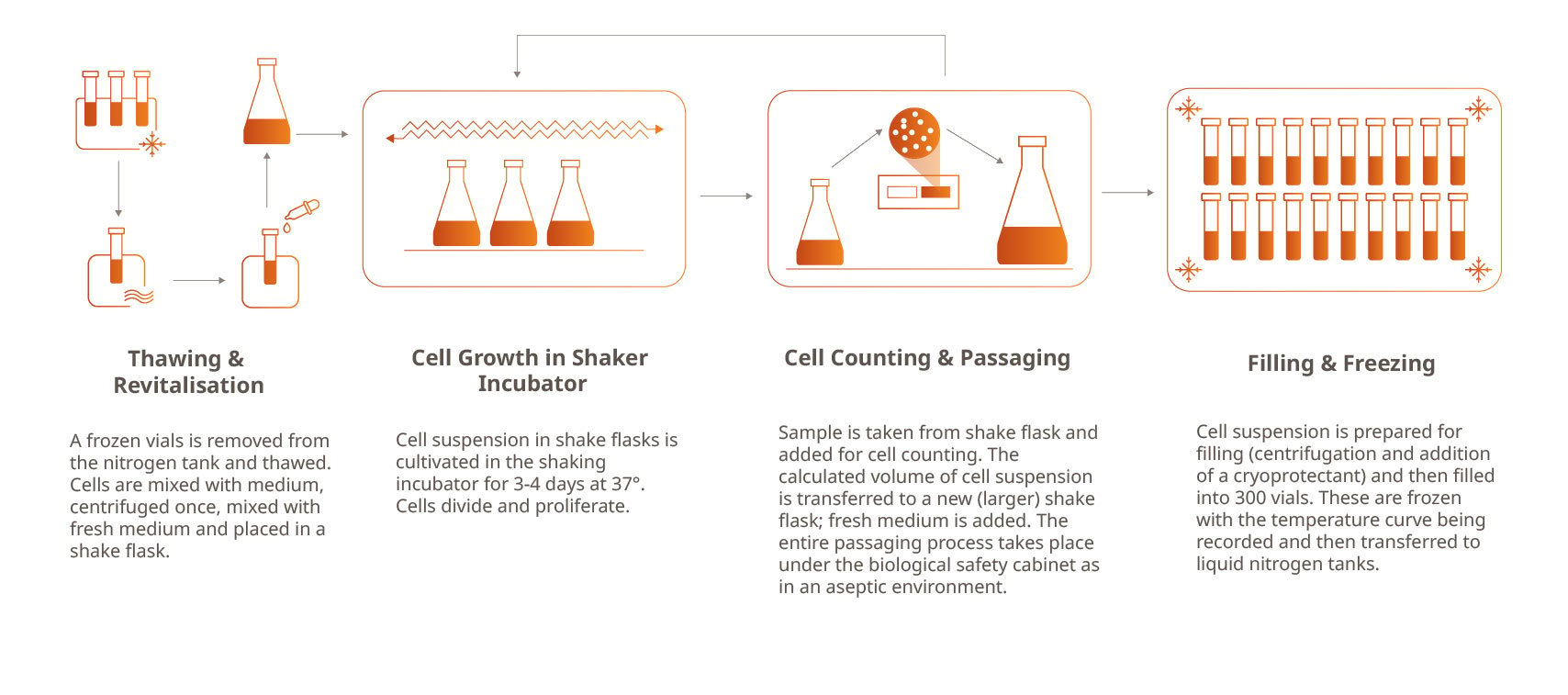
Based on the written documents, a GMP-compliant manufacturing process in a clean room environment is executed. One vial from a defined pre-cell bank is thawed and the cells are revitalized. For about ten to eleven days, the cells are grown in Erlenmeyer flasks in a shaker incubator until the necessary cell count has been reached. Finally, a freezing suspension -containing cells, culture medium, and DMSO as cryo protectant - is mixed and aliquoted into vials. Our trained and experienced staff ensure a homogeneous distribution of the cell suspension across all vials. While the vials are frozen to -80°C, the temperature is recorded. At the end, the vials are transferred into liquid nitrogen tanks for safe long-term storage. All manufacturing steps as well as results of in-process analytics are summarized in a batch record.
Of the 300 vials produced, 250 vials are a deliverable for the customer. Up to 50 vials are used for GMP-compliant testing of the cell banks. Our experienced QC staff tests the cell banks for sterility, mycoplasma, and homogeneity as well as cell growth and viability after revitalization. Together with qualified external CRO partners, our cell banks are further tested for different adventitious agents. All test results are summarized in a test record.
In addition to the production of cell banks, we also offer the generation of high density cryo-seed intermediates (HDCSI). This means larger aliquots (~ 1E8 cells/mL in 100mL – 1000-fold viable cell count of classical MCB), which can be used for direct inoculation of an N-1 seed train bioreactor (25L culture volume). This makes it possible to skip the seed train in shake flasks, which saves time and enhances process safety. This procedure is particularly worthwhile if the production of several batches is planned. The HDCSI are of course manufactured in compliance with GMP in a clean room environment. Up to 30 aliquots can be homogeneously filled from one freezing suspension.
Cell Banking Workflow
Our Promise To You
Save Time to Clinic
- Process for first GMP material possible shortly after storage of MC
- No waiting for complete testing (technical release), which then take place until release of drug substance
Rely on Quality
- Production in clean room area under GMP conditions
- Homogeneous cell banks, up to 300 vials
Get Quick Resupply
- Use of intermediates (HDCSI) saves 14 days (seed train in shake flasks) in a production process





