Advancing Tomorrow’s Medicines
Vision & Mission
For one world in which there is treatment for every disease
Who We Are and What We Do
Guided by our vision and mission, ProBioGen has developed numerous innovative technologies and biopharmaceutical active ingredients for our clients.
We are a recognized leader in contract development and manufacturing organizations (CDMOs) and pioneering technology provision. Our expertise spans the spectrum of cell line engineering, process development, and good manufacturing practice (GMP) manufacturing of biopharmaceuticals, catering to discerning clients in the biopharmaceutical sector. We proudly offer a suite of proprietary, tailored technologies designed to elevate the efficiency and precision of biopharmaceutical manufacturing and analysis. Among others these are proven technologies which are used in approved products.
We also provide process development, GMP manufacturing services, and advanced technologies specifically designed for viral vaccines, as well as innovations in cell and gene therapy (CGT).
~
Employees
Production Sites
Years of Experience
%
Quality
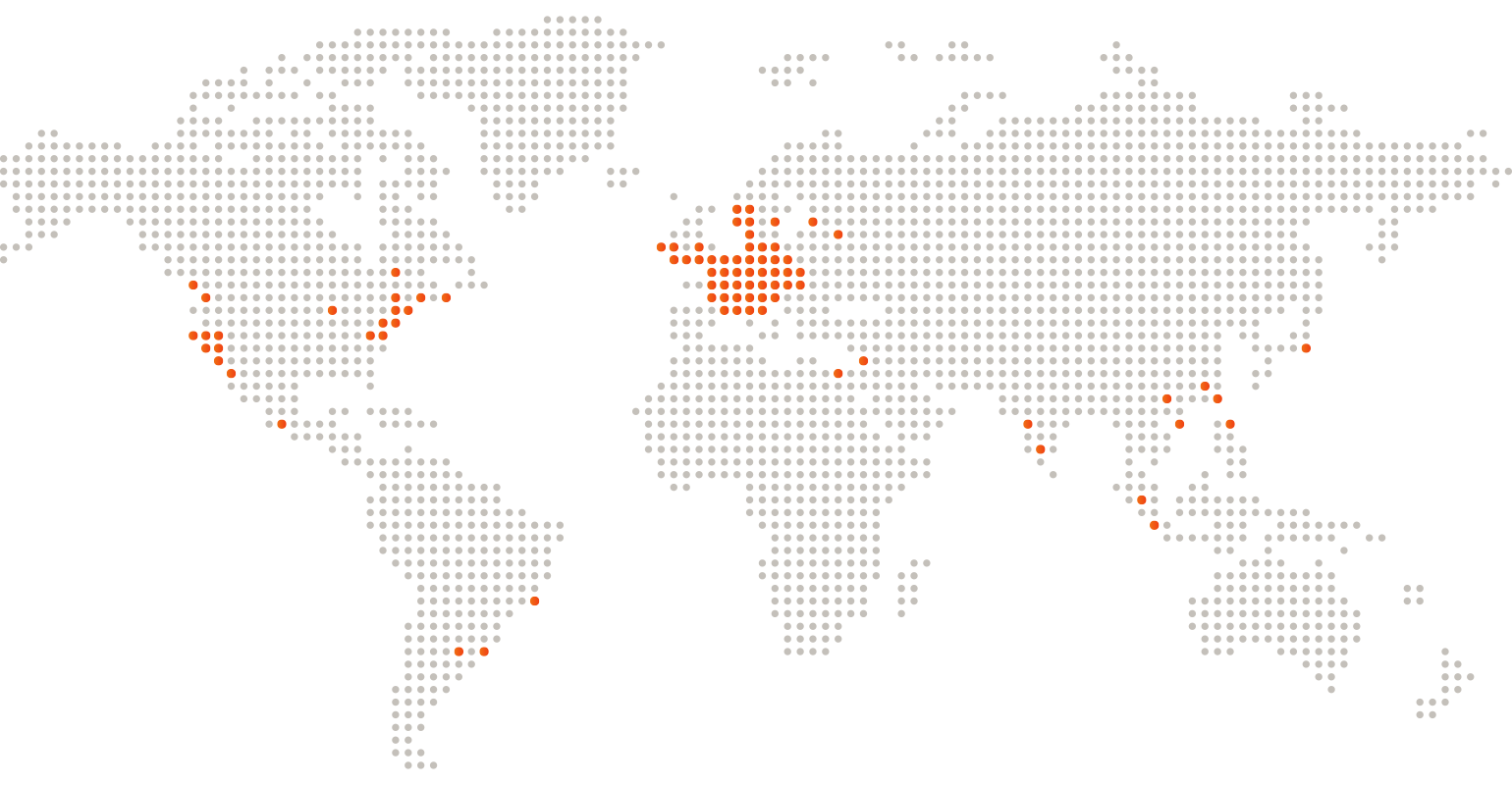
Global Customer Base
Partners & References
The remarkable scientific progress over the last two decades has given rise to the development of new technologies at an unprecedented pace. Our partnerships are collaborative and target specific results, with a strong focus on value for our end customers.

Thermo Fisher Scientific
We engineered the Freedom™ CHO-S™ Kit platform, which enables cost-and-time-efficient stable cell line development for research and commercial purposes, in close collaboration with Thermo Fisher Scientific. The kit is available via Thermo Fisher Scientific.

Boehringer Ingelheim
Boehringer Ingelheim has a non-exclusive license for ProBioGen's proprietary GlymaxX® ADCC enhancement technology (Antibody-Dependent Cell-Mediated Cytotoxicity) to produce antibodies with enhanced ADCC potency.

Thermo Fisher Scientific
We engineered the Freedom™ CHO-S™ Kit platform, which enables cost-and-time-efficient stable cell line development for research and commercial purposes, in close collaboration with Thermo Fisher Scientific. The kit is available via Thermo Fisher Scientific.

Boehringer Ingelheim
Boehringer Ingelheim has a non-exclusive license for ProBioGen’s proprietary GlymaxX® ADCC enhancement technology (Antibody-Dependent Cell-Mediated Cytotoxicity). Under this license, Boehringer Ingelheim applies GlymaxX for third parties from its global contract manufacturing platform as well as to its own development candidates, to produce antibodies with enhanced ADCC potency.
References
Our customer range is extensive; some of our key customers and selected references include:















